Researchers at the Seoul National University College of Engineering have developed a new electrode for water electrolysis that does not use metal catalysts and can heal on its own. This is a major boost for the production of green hydrogen as the world looks for ways to move away from fossil fuels.
As countries race to meet the ‘net-zero’ emission goals, technologies such as wind and solar-based power have gained prominence. These technologies help generate clean electricity but are ineffective for high-power applications such as heavy transport or manufacturing.
Hydrogen is a good alternative to fossil fuels with its high energy density. Still, current production methods are carbon-intensive and detract from our net-zero ambitions.
Electrolysis of water is one of the simplest approaches to generating hydrogen, and it can be powered by clean electricity to achieve this in an emission-free way. The approach, however, relies on catalysts made using precious metals, which makes the process expensive.
By switching to catalyst-free electrodes, researchers in Korea have made a major advance in green hydrogen production.
How does it work?
The research team led by Jeyong Yoon and Jaeyune Ryu, both professors at the Department of Chemical and Biological Engineering at Seoul National University, developed a novel approach where only nickel was used to make the electrodes.
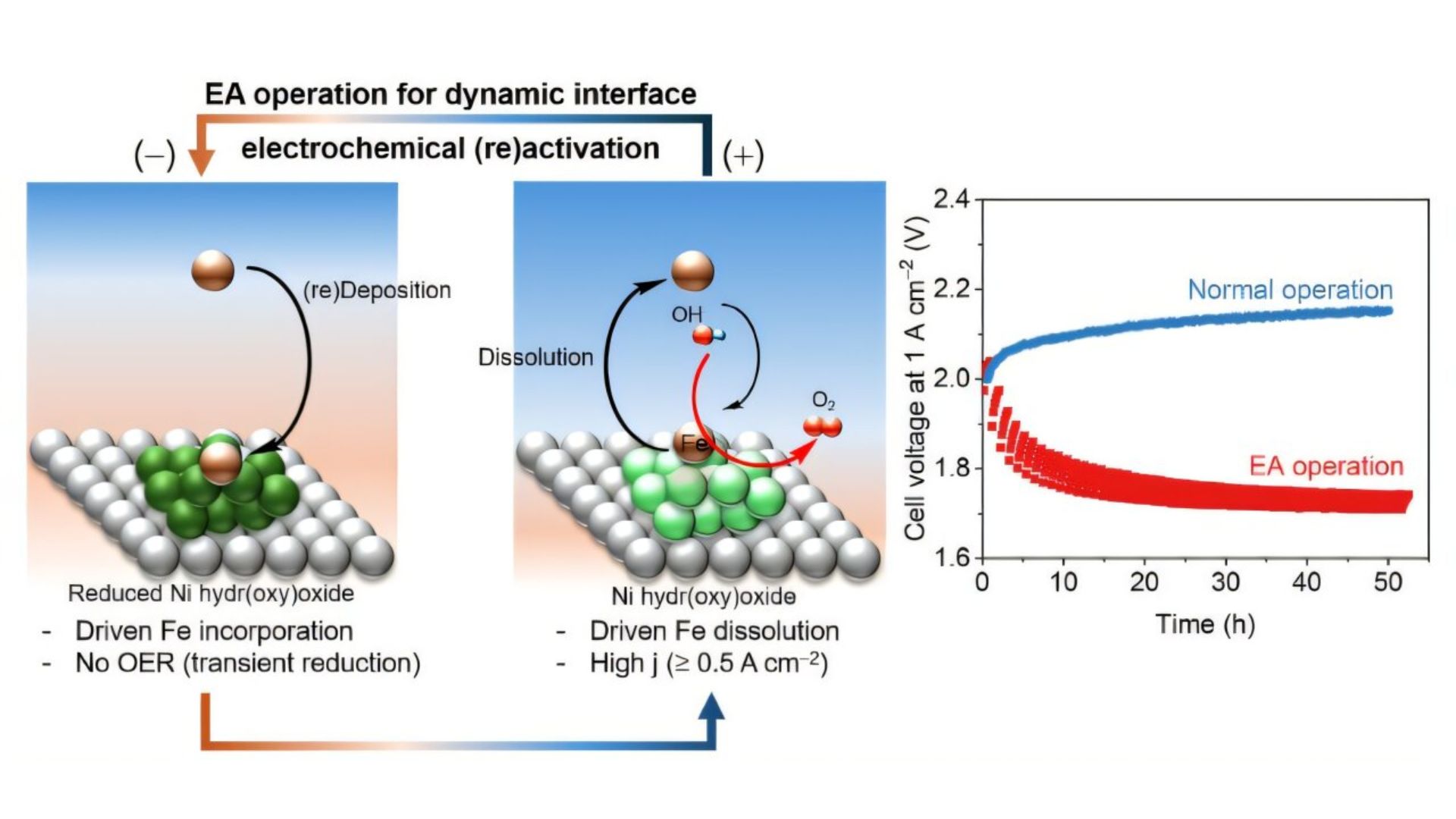
Typically, these electrodes are coated with metal catalysts that degrade over time and must be replaced, making the process expensive. Moving away from a catalyst-dependent approach helps reduce the cost of production; however, the electrode is still prone to degradation over time.
To resolve this, the researchers used a method dubbed dynamic polarization controls, allowing the electrode a brief rest period. By applying a weak reducing voltage to the electrode, the researchers allowed trace amounts of Fe dissolved in the electrolyte solution to reattach to the electrode surface.
The iron then bonds with Ni to make a highly active oxygen evolution catalyst layer on the electrode, improving the reaction performance and giving it a self-healing mechanism.
Inexpensive and scalable approach
The researchers demonstrated their approach through over 1,000 hours of stable electrolysis cell operation, even under a high current density of 1 A per sq. cm. To further demonstrate that the success was not limited to a laboratory-scale setup, the researchers set up a three-stack cell system with a 25 sq. cm active area per cell. They ran it successfully for several hundred hours.
“This research is not just about optimizing operating conditions,” said Jaeyune Ryu, who co-led the research. “It is a fundamental application study that systematically clarified the complex electrochemical interactions at the electrode/electrolyte interface and successfully implemented them in a real system. It is a case where both theoretical insight and industrial relevance were effectively demonstrated.”
The approach will help produce growth in hydrogen and help Korea transition to a hydrogen-based economy, which the country has designated as one of the 12 core approaches for achieving carbon neutrality and leading the world in a leading approach.
“A hydrogen production method that does not rely on catalysts is a transformative strategy that can greatly improve the economics and scalability of green hydrogen,” added Jeyong Yoon in the press release. “This achievement marks a real turning point for hydrogen economy technologies aimed at carbon neutrality.”




